Recent Advances in Treatment of Stage IV Metastatic Non-Small Cell Lung Cancer
Non-small cell lung cancer (NSCLC) is the most common type of lung cancer. Approximately 40% of NSCLC patients are diagnosed with stage IV after the tumor has metastasized. As NSCLC is less sensitive to chemotherapy compared to small cell lung cancer (SCLC), research focus has focused on the development of immunotherapies using monoclonal antibodies (mABs).
What are anti-EGFR mABs and TKIs?
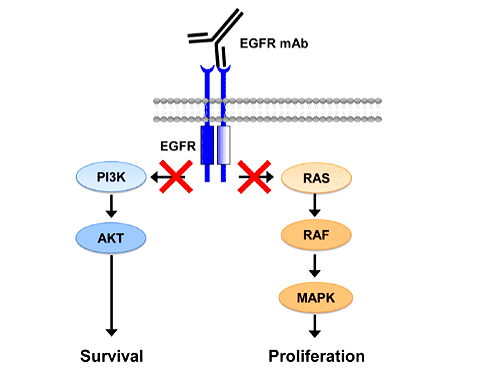
EGFR, also known as erbB-1, is a 170-kDa member of the family of erbB protein kinase receptors. It consists of an extracellular ligand-binding domain, a transmembrane domain, and an intracellular tyrosine kinase domain. Binding with the 133-kDa epidermal growth factor (EGF) polypeptide or the 17-kDa transforming growth factor alpha (TGF-α) polypeptide activates the EGFR and its downstream signal transduction cascades as the MAPK, Akt and JNK pathways leading to cellular differentiation, mobility, proliferation, and survival. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics has shown that the EGFR gene (Ensembl Gene ID: ENSG00000146648) is widely expressed in the lung tissue with 15.61 RPKM/FPKM unit of transcript expression. Somatic mutations of EGFR gene lead to overexpression and constant activation of EGFR, uncontrolled cell division, and carcinogenesis. The comprehensive molecular profiling by Cancer Genome Atlas Research Network has shown that a number of cancers are associated with drug sensitizing mutations and overexpression of EGFR including 15%-20% of NSCLC adenocarcinoma subtype.
Anti-EGFR mABs are large antibodies with monovalent affinity produced by recombinant DNA technology. The mechanism of action of anti-EGFR mABs is blocking the extracellular ligand-binding domain of EGFR thus attenuating the neoplastic actions of EGF. In addition, the anti-EGFR mAB-EGFR complex is internalized and destroyed by the target cell, thus reducing the number of available EGFRs for recycling back to the cell membrane. TKIs are relatively smaller molecules compared with the Anti-EGFR mABs. The mechanism of action of TKIs is inhibiting phosphorylation of the intracellular tyrosine kinase domain of EGFR. It was clear that anti-EGFR mABs and TKIs could have a significant role in treatment of NSCLC. Translational research became a necessity to translate hypothesis into evidence-based medicine.
The role of translational research in the drug development process of anti-EGFR mABs and TKIs for stage IV metastatic NSCLC:
Proof of Mechanism Studies:
“Proof of mechanism” studies are pre-clinical drug development studies. They are conducted in the research laboratory to accept or reject a hypothesis that a drug candidate, through its interaction with its molecular target (receptor or enzyme), will affect the signalling cascade, interfere with the molecular pathway, and intentionally alter the biology of the target cells.
The corresponding landmark “proof of mechanism” studies in the drug development process of anti-EGFR mABs and TKIs for stage IV metastatic NSCLC include:
- A study by Sordella R, Bell DW, et al., published in 2004, which showed the inhibitory effect of a TKI known as Gefitinib on the Akt and STAT signalling pathways transduced by mutant EGFR in NSCLC cell lines.
- A study by Turke AB, Zejnullahu K, et al., published in 2010, which showed that MET amplification in EGFR mutant NSCLC activates ERBB3/PI3K/AKT signalling cascade and causes resistance to TKIs.
Proof of Concept Studies:
“Proof of concept” studies “also known as phase I and phase II studies” are early stage clinical drug development studies. They are conducted on a small scale of human subjects to show the effect of the investigational medicinal product (IMP) on the disease and find the recommended dose for “proof of principle” and “proof of efficacy” studies.
The corresponding landmark “proof of concept” studies in the drug development process of anti-EGFR mABs and TKIs for stage IV metastatic NSCLC include:
- A phase I study by Modjtahedi H, Hickish T, et al., published in 1996, which showed the safety outcomes of anti-EGFR monoclonal antibody ICR62 in NSCLC.
- A phase II study by Pérez-Soler R, Chachoua A, et al., published in 2004, which showed the clinical effect and objective response of Erlotinib in EGFR-expressing NSCLC
Proof of Principle Studies:
“Proof of principle” studies are early stage clinical drug development studies. They are conducted on a small scale of human subjects between phase I and phase II studies to evaluate the effect of the IMP on disease biomarkers (prognostic, predictive, efficacy, safety, surrogate).
The corresponding landmark “proof of principle” studies in the drug development process of anti-EGFR mABs and TKIs for stage IV metastatic NSCLC include:
- A study by Maheswaran S, Sequist LV, et al., published in 2008, which showed that the emergence of T790M mutation in circulating NSCLC cells was predictive of drug resistance to TKIs.
- A study by Hirsch FR, Varella-Garcia M, Southwest Oncology Group, et al., published in 2005, which showed that the increased EGFR gene copy number detected by fluorescence in situ hybridization is associated with increased sensitivity to Gefitinib in patients with NSCLC bronchioloalveolar carcinoma subtype.
Proof of Efficacy Studies:
“Proof of efficacy” studies “also known as phase III studies” are late stage clinical drug development studies. They are conducted on a large scale of human subjects to evaluate the effect of the IMP on the primary and secondary clinical endpoints.
The corresponding landmark “proof of efficacy” studies in the drug development process of anti-EGFR mABs and TKIs for stage IV metastatic NSCLC include:
- An open randomised phase III study by Pirker R, Pereira JR, et al., published in 2009, which compared Cisplatinum and Vinorelbine regimen plus Cetuximab with Cisplatinum and Vinorelbine regimen alone in patients with advanced EGFR-positive NSCLC.
- An open label randomised phase III study by Douillard JY, Kim E, et al., published in 2007, which compared Gefitinib with Docetaxel in patients with locally advanced or metastatic NSCLC pre-treated with platinum-based chemotherapy.
The significant role of anti-EGFR mABs iand TKIs n treatment of stage IV metastatic NSCLC:
Survival statistics of stage IV metastatic NSCLC has favourably evolved in the past few years. This is mostly due to the recent advances in NSCLC targeted therapy and the significant role of anti-EGFR mABs and TKIs. Examples of anti-EGFR mABs and TKIs that were developed in the past few years and have proven efficacy in treatment of stage IV metastatic NSCLC include Cetuximab, Necitumumab, Erlotinib, Gefitinib, and Afatinib. Based on data collected from the National Cancer Institute (NCI) Surveillance, Epidemiology and End Results program (SEER), the relative 5 years survival rate for cases of stage IV metastatic NSCLC has doubled in the last 30 years. The interplay between basic laboratory research and applied clinical research caused a paradigm shift in the natural history of many incurable diseases, including lung cancer, giving hope for a better tomorrow.
Your suggestions on how to improve and develop our medical writing service are welcomed. Please take a moment and provide your valuable feedback. Thank you!